
Ever wonder why some drugs have two names? One that sounds like a chemical code, and another that’s easy to remember, like Advil or Prozac? That’s because every medicine has three names: a brand name, a generic name, and sometimes two different generic names depending on where you are in the world. This isn’t just confusing-it’s a safety system. And at the heart of it are two major naming bodies: the USAN and the INN.
What Are USAN and INN?
USAN stands for United States Adopted Names. It’s the official system used in the United States to give every drug a standardized, nonproprietary name. Think of it as the government’s way of saying, ‘This is what we call this chemical, no matter who makes it.’ The USAN Council, made up of experts from the American Medical Association, the US Pharmacopeia, and the American Pharmacists Association, has been doing this since 1964.
INN stands for International Nonproprietary Name. It’s the global version, run by the World Health Organization since 1950. Its job? Make sure doctors in Tokyo, Berlin, or São Paulo all know exactly what drug they’re talking about. The goal is simple: reduce confusion, prevent errors, and keep patients safe.
Both systems avoid trademarked names. That means you won’t see something like ‘Pfizer’s Pain Relief’ as a generic name. Instead, you get something like ibuprofen-a name that belongs to everyone, not just one company.
How Do These Names Actually Work?
It’s not random. There’s a science to it. Most generic names follow a pattern: a made-up prefix, plus a meaningful suffix called a ‘stem.’ The stem tells you what the drug does.
Take omeprazole. The ‘-prazole’ part? That’s the stem. It tells you it’s a proton pump inhibitor-a drug that reduces stomach acid. Same with atorvastatin. The ‘-statin’ ending means it’s a cholesterol-lowering drug. You don’t need to memorize every drug. Just learn the stems, and you can guess the class.
For monoclonal antibodies, it gets even more specific:
- -mab = monoclonal antibody
- -ximab = chimeric (part mouse, part human)
- -zumab = humanized (mostly human)
- -tumab = targets tumors
So if you see rituximab, you instantly know it’s a chimeric antibody used to treat cancer or autoimmune diseases. That’s powerful. It helps doctors make faster, safer decisions.
Why Do Some Drugs Have Two Generic Names?
Here’s where things get messy-and dangerous.
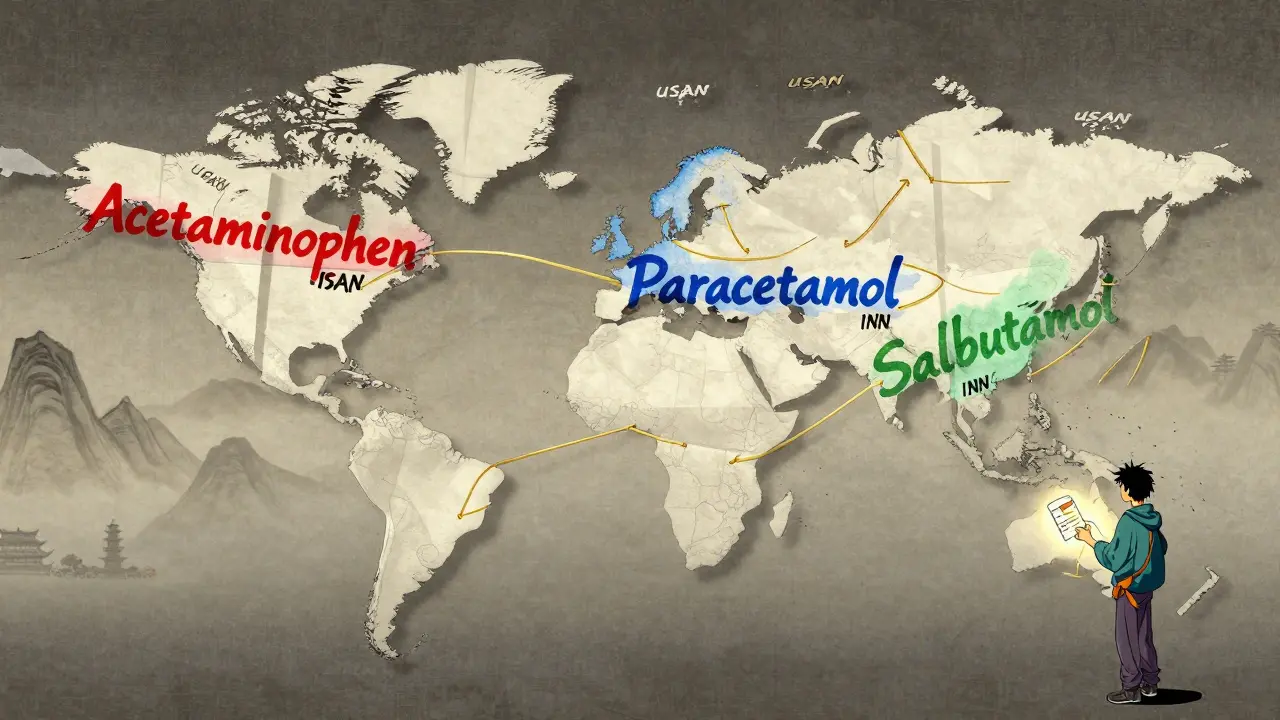
Acetaminophen in the U.S. is called paracetamol everywhere else. Albuterol is salbutamol. Rifampin is rifampicin. These aren’t typos. They’re official differences between USAN and INN.
Why? History. The U.S. developed its own naming system before fully aligning with global standards. Some names stuck because they were already common in American medical practice. The USAN Council doesn’t treat INN names as automatic defaults. They make their own calls, even if it means creating a slight mismatch.
The problem? Medication errors. A nurse in Canada might prescribe salbutamol. A patient from the U.S. might show up with a prescription for albuterol. If the pharmacist doesn’t know they’re the same thing, the patient gets the wrong dose-or none at all. Studies show these kinds of mix-ups happen more often than you’d think, especially in hospitals with international staff or travelers.
And it’s not just names. Stereoisomers add another layer. Esomeprazole is the S-isomer of omeprazole. The ‘es-’ prefix tells you it’s a specific version of the molecule. That’s intentional. It helps doctors know exactly which form they’re prescribing.
How Are These Names Created?
It’s not a quick process. When a drug company develops a new molecule, they don’t just pick a name. They go through a long, strict procedure.
First, they submit up to six name options to both the USAN and INN committees. These names are checked against every existing drug name-brand and generic-across the globe. They’re tested for pronunciation, spelling, and how easily they could be confused with other drugs.
For example, if a company suggests ‘Zoliprine,’ the USAN team will check: Is that too close to ‘Zoloft’? ‘Lipitor’? ‘Prinivil’? What about in Spanish or Mandarin? Even small similarities can lead to deadly errors.
On average, companies go through 15 to 20 name ideas before finding one that clears the system. About 30-40% of submissions get rejected or need revisions. The whole process takes 18 to 24 months-sometimes longer than the clinical trials themselves.
And here’s the kicker: 65% of the drugs that get a USAN name never even make it to market. They fail in trials. But their names stay on file, just in case.
Why Does This Matter for Patients?
Every year, medication errors tied to confusing drug names cost the U.S. healthcare system around $2.4 billion. That’s not just money. It’s hospital stays, missed work, and sometimes lives lost.
Generic names are meant to be clear, consistent, and safe. When a doctor writes ‘metformin,’ they’re not thinking about the brand ‘Glucophage.’ They’re thinking about the chemical that lowers blood sugar. That’s the point.
But when two countries use different names for the same drug, it breaks that clarity. A patient traveling abroad might not recognize their own medication. A pharmacist in a rural clinic might not have access to international databases. That’s why the WHO and USAN Council are pushing harder than ever for alignment.
Today, 95% of USAN and INN names match. But those 5%? They’re the ones that cause trouble.

What About Brand Names?
Brand names are a different beast. They’re marketing tools. They’re catchy. They’re easy to remember. ‘Lipitor,’ ‘Zoloft,’ ‘Humira’-these names are designed to stick in your mind, not to tell you how the drug works.
But here’s the rule: brand names must never look or sound too much like a generic name. The FDA and WHO won’t approve a brand name like ‘Prozacine’ if there’s already a drug called ‘Prozac.’ That’s trademark infringement and a safety risk.
Brand names also can’t imply effectiveness. You won’t see a drug called ‘CureAll’ or ‘InstantRelief.’ That’s false advertising. The name has to be distinctive, not misleading.
That’s why you see names like ‘Cialis’ or ‘Seroquel’-they’re unique, pronounceable, and legally safe. But they tell you nothing about the drug’s function. Only the generic name does that.
What’s Next for Drug Naming?
As new types of drugs emerge-gene therapies, RNA treatments, antibody-drug conjugates-the old stem system is being pushed to its limits. How do you name a drug that edits your DNA? Or delivers medicine directly to cancer cells using a virus?
The WHO and USAN Council are already working on updates. In 2021, they revised the rules for monoclonal antibodies to include newer formats. They’re now building frameworks for these emerging therapies.
One thing’s certain: the system will keep evolving. But the goal stays the same. Safety first. Clarity always. No matter how complex the science gets, the name must still be easy to read, say, and understand-especially when someone’s life is on the line.
What You Can Do
If you’re a patient:
- Always ask for the generic name of your prescription.
- Compare the name on your bottle to the one your doctor wrote down.
- If you’re traveling, bring a list of your meds with both brand and generic names.
- Use apps or tools that show you the INN and USAN equivalents.
If you’re a healthcare worker:
- Teach your team the common stems. It saves time and prevents errors.
- Double-check names when prescribing to international patients.
- Don’t assume ‘albuterol’ and ‘salbutamol’ are different drugs.
Drug naming isn’t just bureaucracy. It’s a silent guardian in every hospital, pharmacy, and clinic. It’s the reason you can trust that your medicine is what it says it is.
Why do some drugs have different names in the U.S. and other countries?
The U.S. uses USAN (United States Adopted Names), while most other countries use INN (International Nonproprietary Names). While 95% of these names match, a few differ due to historical usage or regional preferences. For example, acetaminophen (U.S.) is paracetamol elsewhere. These differences exist because the USAN Council prioritizes U.S. medical practices, even when they don’t fully align with global standards.
How are generic drug names chosen?
Drug companies submit up to six name options to the USAN and INN committees. These names are checked for conflicts with existing drugs, pronunciation, spelling, and potential for confusion. The committees look for a meaningful stem that indicates the drug’s class (like ‘-prazole’ for acid reducers) and a unique prefix. Names must be original, safe, and not trademarkable. The process takes 18-24 months and often involves multiple revisions.
What’s the purpose of drug name stems like ‘-mab’ or ‘-statin’?
Stems are the ending parts of generic names that tell you the drug’s class or mechanism. For example, ‘-statin’ means it lowers cholesterol, ‘-prazole’ means it reduces stomach acid, and ‘-mab’ means it’s a monoclonal antibody. This helps doctors quickly identify what a drug does, even if they’ve never seen the name before. It’s a built-in safety feature.
Can a brand name be the same as a generic name?
No. Regulatory agencies like the FDA and WHO strictly forbid brand names from sounding too similar to generic names. For example, a company can’t name a drug ‘Ibuprofen’ as a brand-it’s already the generic name. Brand names must be distinctive and not misleading. They’re meant to be memorable for marketing, not to describe the drug’s function.
Do generic names ever change after a drug is approved?
Rarely. Once a drug gets its USAN or INN, the name stays the same-even if its use changes. For example, ‘-prazole’ drugs were originally labeled as antiulcer agents, but now they’re used for GERD and other conditions. The name doesn’t update to reflect new uses because consistency matters more than accuracy in indication. Changing names would create confusion and risk errors.
How do naming systems handle new types of drugs like gene therapies?
Traditional stems don’t fit gene therapies or RNA-based drugs because they don’t work like typical small molecules or antibodies. The WHO and USAN Council are developing new naming frameworks for these advanced therapies. While no official system is in place yet, experts are working on stems that reflect delivery methods, target cells, or genetic mechanisms. The goal is to keep the naming system useful, even as science evolves.
So let me get this straight-my ibuprofen is called 'paracetamol' in India, but wait, no, that's not right... oh wait, that's acetaminophen? 😅 This whole system feels like someone dropped a dictionary into a blender and called it 'global health.'
The stem system is brilliant. -statin -prazole -mab. You learn five suffixes and you can guess the mechanism of 80% of drugs. No magic just logic. Simple elegant safe.
I love this so much 💖 Like a secret code for doctors and pharmacists. I used to think drug names were just marketing nonsense but now I get it-there’s science in the chaos 🧪✨
The fact that 65% of USAN names are for drugs that never even hit the market is wild. Like, they spent two years naming a ghost pill. The bureaucracy is its own pharmaceutical subplot. 🤯
This is one of those systems you never notice until something goes wrong. Then you realize it's the quiet backbone of every prescription you've ever taken. Respect to the committees doing the unglamorous work.
I mean, think about it-this whole thing is a monument to human absurdity. We’ve got PhDs spending years debating whether a drug should be called 'flumoxine' or 'flumoxene' because in Czechoslovakia in 1972, someone mispronounced it and now we're stuck with it. And don't even get me started on the fact that 'es-omeprazole' is literally just the left-handed version of omeprazole. We're naming molecules like they're Olympic gymnasts.
I had a friend once get hospitalized because her doctor wrote 'salbutamol' and the pharmacy gave her 'albuterol' and she thought it was a different drug. She didn't take it for three days. I swear to god this is the most dangerous bureaucracy in medicine.
The stems are just a placebo for safety. Real change would be AI-driven naming. Or maybe just letting pharma companies pick names like 'VibraCure 9000' and calling it a day. Who even reads these anyway?
It's fascinating how a system designed to prevent death is also responsible for decades of linguistic fragmentation. The fact that we still have acetaminophen vs paracetamol in the 2020s is less about science and more about national pride. We’re all just stubborn children with lab coats.
You people act like this naming system is some sacred artifact. It’s just corporate legalese dressed up as science. The real reason they don’t unify names? Because it’d cost too much to retrain everyone. Profit > safety. Always.
I just learned that -mab means monoclonal antibody and now I feel like a genius 😭 I'm gonna start guessing drug classes at the pharmacy like I'm on Jeopardy! -zumab = humanized? YES! I GOT IT!
I never knew the name of my blood pressure med was just a code. Now I look at it and see -olol and I'm like ohhh beta blocker. Small wins.
I appreciate the depth of this explanation. The fact that names are vetted across languages and potential mispronunciations speaks to a level of diligence that is rarely acknowledged. It's not glamorous, but it's essential. Thank you for highlighting this quiet infrastructure.